Dare to disrupt
Rethink patient recruitment with SubjectWell
Powered by a robust technology platform, the largest Patient Network, and an extensive partner network, we solve the greatest challenges in patient recruitment.
Introducing the SubjectWell+ platform
The SubjectWell+ platform delivers the most comprehensive solutions for patient recruitment and site enablement, seamlessly paired with advanced commercial capabilities to connect patients with clinical trials and new treatments. Powered by robust technology, a Patient Network of 13 million+ patients, services, creative expertise, and an extensive partner network, SubjectWell+ is your partner for change.
For patient recruitment and commercial solutions, it’s time for more. It’s time for SubjectWell.

Innovative patient recruitment and commercial solutions
At SubjectWell, we collaborate with every key player in clinical research and commercial development to accelerate patient recruitment, improve site performance, optimize study outcomes, and drive market success. Whether you’re a sponsor, CRO, site, site network, or brand marketer, our disruptive solutions meet your unique needs and deliver measurable results.
Explore how our solutions advance healthcare breakthroughs and help you achieve commercial excellence.
It’s time to transform patient recruitment
The clinical trial landscape has changed dramatically. Protocols are more complex, patient needs have evolved, and regulatory standards are ever-changing. Globalization has made finding patients even more challenging. Yet, patient recruitment strategies have remained stagnant, leading to an 80% clinical trial failure rate and $40 billion in unrecoverable losses. (BioPharma Dive. January 29, 2019.)
-
30%
Accelerate enrollment by
$40B
Recoup $40B in losses
13M+
patients in our network
-
1,400+
studies completed
8,800+
sites served
100+
clinically-trained patient companions
-
22
languages supported in our Companion Centers
600+
therapeutic areas
Expansive global recruitment across all therapeutic areas
Rare, complex, or chronic conditions
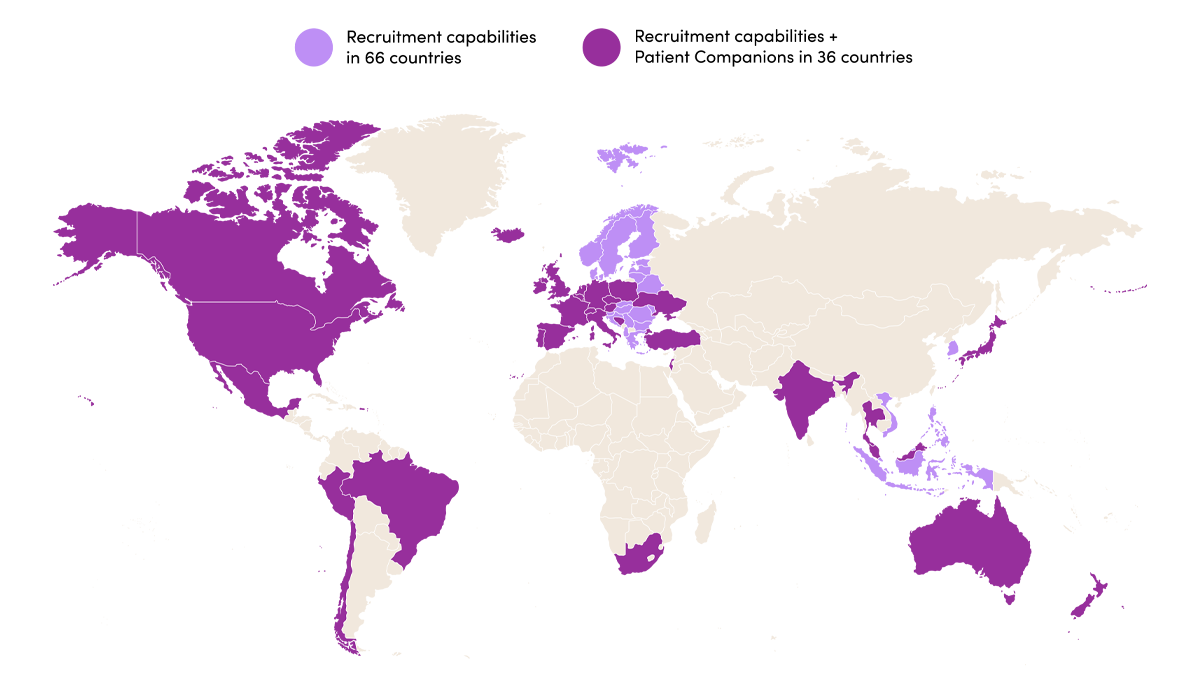
SubjectWell can recruit patients for clinical trials in 102 countries and provides Patient Companions across 36 countries. From rare disease and oncology to chronic conditions and vaccine studies, our expertise ensures your study’s success across all therapeutic areas. With over 13 million patients in our Patient Network, we’ve supported more than 500,000 patient referrals across 8,800+ sites, delivering measurable results for sponsors and studies worldwide.
Reducing site and patient burden
Clinical trials require trust and understanding as patients make significant sacrifices and navigate difficult decisions. At the same time, sites face increasing challenges such as administrative tasks, resource constraints, and data management burdens, all of which can detract from patient care.
SubjectWell removes these burdens with complementary services that ensure patients stay informed, engaged, and valued throughout the recruitment and trial.
-
Full-Site Support
Our most comprehensive service for relieving site burden. Clinically trained and multilingual Patient Companions provide comprehensive administrative support, including patient communication, transportation logistics, Medical Records Retrieval and review, phone screening to assess patient eligibility, and Site Visit Scheduling.
-
Patient Care & Trial Retention
SubjectWell provides end-to-end support for patients throughout the clinical trial process through study-close.
-
Virtual Waiting Room
If your sites are overloaded or not yet ready, Virtual Waiting Room helps keep patients engaged and prepared. Manage complex systems like CAR-T, lab result dependencies, or flare-ups while reducing dropouts and improving the overall patient experience.
-
Recruitment Simulation
Gather insights that improve the patient experience during patient recruitment process.
-
Secondary Screening
Reduces site effort and time for sites to arrange follow-up with referrals. SubjectWell's Patient Companions schedule the site screening call for patients.
-
Translations
Ease global and multi-country study onboarding with translation of IRB/EC-approved English trial recruitment materials to language(s) needed.
Insights from industry leaders
Stay informed with expert insights on clinical trial trends, patient recruitment strategies, and healthcare innovations.
-

How global standards of care data shape patient recruitment
Treatment patterns, diagnostic pathways, and healthcare access determine who is eligible,03 Mar 2026●6 min -

The future of global patient engagement starts with experience
Why patient experience will define the next era of clinical research18 Feb 2026●4 min -

Why global trials need local trust
Local expertise, human support, and cultural understanding are what turn global studies into10 Feb 2026●4 min -

SubjectWell + Clariness: Why this merger matters
SubjectWell CEO Fred Martin shares his thoughts on what this new chapter means for patient27 Jan 2026●4 min
Get started with SubjectWell
Patient-centricity is paramount. Breakthroughs are in demand. Let’s change patient recruitment and commercial development together.









